Leveraging the EHR to Automate Biosimilar Selection and Streamline the Prior Authorization Process (February 2025)
Author(s):
Biologic drugs are at the forefront of medicine, offering patient benefits and supporting precision medicine efforts.1 As a diverse category of products, biologics are generally large, complex molecules that are often more complicated to characterize than are small-molecule drugs. For patients with hematologic malignancies and solid tumors, the introduction of novel therapies in the form of monoclonal and other biologic drugs has been important to improving patient care.1,2 Biologics include reference products, biosimilar products, and interchangeable biosimilar products.3 These new therapies provide more treatment options, but they come with increasingly high costs.1 The response of industry partners to the increasing cost of drug development has been the introduction of more economically developed biologics in the form of biosimilars.1 Use of biosimilars may save the US health system $54 billion from 2017 to 2026.4 Accordingly, biosimilars have gained a significant market share in many therapeutic areas. Health care savings from the use of these biologic therapies reportedly climbed to $3 billion in the second quarter of 2022 for a total of $18 billion over the past 6 years.4,5
Biosimilars and Reimbursement
A reference product is the single biological product approved by FDA based on a full complement of safety and efficacy data. Data on a proposed biosimilar or interchangeable biosimilar are compared against information on this reference product. A biosimilar is derived from living systems; each dose usually results from a mixture that is biologically comparable to an already approved FDA-approved reference product. Clinical studies are conducted to compare the proposed biosimilar with the reference product and show that there are no clinically meaningful differences in terms of safety and efficacy.3,6
The preferred lists of biosimilars are not uniform among various payers, making it challenging for health care providers and facilities to navigate and resulting in patient delays, insurance denials, and patient and provider frustrations.
One of the main challenges associated with the increased use of biosimilars in health care is the complex prior approval and step-therapy process for insurance payers.7,8 Payers use prior authorization to review and assess the appropriateness and medical necessity of therapy and the efficient use of health care services and procedures under the provisions of patient health care benefits.7,8,9 These processes require that health care providers obtain advanced approval to ensure that a health plan will cover a specific product before it is delivered to the patient.10 Step therapy is another management tool that requires patients to try specific therapies before other treatments are approved by a health insurer. Both utilization management tools require extensive paperwork, long phone calls, and coordination between physicians, pharmacists, and insurance representatives.11-14 For patients, delays caused by prior authorization denials often result in treatment delays, which exacerbate health conditions and increase anxiety. While prior authorization and step therapy are increasingly used to support appropriate use of medications and manage associated costs, provider and patient challenges related to administrative burden and access to care are also on the rise. The bottom line is that the overall impact of these administrative burdens strains the health care system and undermines the efficiency and effectiveness of patient treatment and care.15
In health insurance, a formulary is defined as a list of medications covered by a specific insurance payer. In the formulary management process, biosimilars are thoroughly assessed for clinical effectiveness and value before being added to an insurance company’s coverage list, which ultimately determines whether biosimilars will be included in the health insurance formulary.16 The health insurer will then develop a list of preferred biosimilar and/or reference products for biologics that is unique to that payer’s formulary. The preferred lists of biosimilars are not uniform among various payers, making it challenging for health care providers and facilities to navigate and resulting in patient delays, insurance denials, and patient and provider frustrations.
Identifying the Problem
At the introduction of biosimilars to the marketplace, The Ohio State University Comprehensive Cancer Center—Arthur G. James Cancer Hospital and Richard J. Solove Research Institute (The James) took a reactive approach to preferred biologic medications. Treatment plans entered with reference products were submitted for prior authorization to health insurers. Health payers that did not allow the reference products returned denials for the requests and communicated their accepted preferred biosimilar. In turn, the provider and/or pharmacist had to change the treatment plan to include the preferred biosimilar and submit a new prior authorization for the patient. This process not only required extra steps for the provider and/or pharmacist, but it also delayed the start of therapy for the patient.
To decrease this administrative burden, The James initiated a process for provider selection of preferred biosimilars. This new process was modeled after a pharmacist-led biosimilar program at Mayo Clinical Foundation, which involved creation and use of a payer preference list.17,18 At The James, at the time of order entry, pharmacists reviewed a list of preferred medications by insurers and intervened when necessary to change the order to a preferred biosimilar. Due to the manual nature of this intervention, delays and denials still occurred; importantly, they were associated with significant administrative burdens for the provider, pharmacist, and prior authorization teams. These ongoing challenges led The James to seek a solution that would:
- Remove the administrative burden of duplicative work for pharmacists and providers
- Leverage the capabilities of the electronic health record (EHR)
- Increase the use of biosimilars
- Reduce the turnaround time between prior authorization and initiation of treatment.
The James leveraged the unique skill of informatic pharmacists to create an EHR-based tool that would automate selection of preferred products (biosimilars) by payer while considering hospital formulary and contractual preferences. Here is how it was done.
Part 1. Development of an EHR Solution
The James pharmacy department began by forming a steering committee to formulate a plan for the biosimilar intervention and introduce an EHR implementation tool. Members of the steering committee worked in finance, pharmacy informatics, oncology infusion, prior authorization, clinical oncology, and drug information. At the time of the pilot planning process, The James’ EHR parent system, Epic (Epic Systems), did not have a recommended pathway for automatic product selection. The steering committee initially focused on biosimilars in the hematology and oncology setting due to their high use and the presence of a large payer mix with varied preferred products.
The first step was to evaluate a rule-based information technology when treatment orders were placed that would align with the patient’s insurance company’s preferred biosimilar medication. Within the EHR, our pharmacist informaticists presented 2 options for biosimilar selection: rule-based advanced order groups or rule-based orderable records. One of the major differences between the 2 workflows is related to rule evaluation for product selection.
The advanced order group is a rule-based function in the EHR that provides the physician with options for an available treatment pathway when placing an order. The advanced order group only works when the treatment plan is initially placed. A limitation of the advanced order group is that changes in insurance or payer preferences after the treatment plan is placed will not update or auto-populate for existing plans or patients.
For an orderable record, the rule is evaluated every time a medication is dispensed. Within the orderable record, the products are interchangeable. Therefore, a new insurance authorization is not triggered when the rule selects a product that is different from an agent that was dispensed to the patient previously.
Due to the financial risk associated with missing insurance authorizations, The James selected the advanced order group option.
Part 2. Development of a Financial Tool
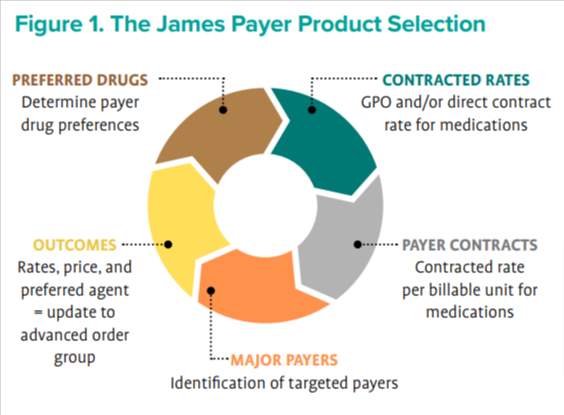
The next step was to evaluate the reference and biosimilar agents to employ for the pilot with the advanced order group. Identification and verification of preferred biosimilars was conducted by researching the published literature available on each payer website. From this research, it was determined that The James’ top 25 contracted payers would impact a large percentage of the health system’s then–payer mix. The team worked with the managed care department to obtain the contracted reimbursement rate per billable pharmaceutical unit and reviewed contracting purchasing rates for all biosimilar and reference products. The pharmacy business operations department developed a proprietary calculator that compiled the preferred biosimilars and references medications, contracted rates, and purchase rates to determine a preferred agent to be chosen as the primary medication (biosimilar or reference) choice for these 25 payers. Once the proprietary calculator determined the preferred agent, the advanced order group rule was populated with the selected biosimilar (Figure 1).


Part 3. Implementation of the New EHR Tool
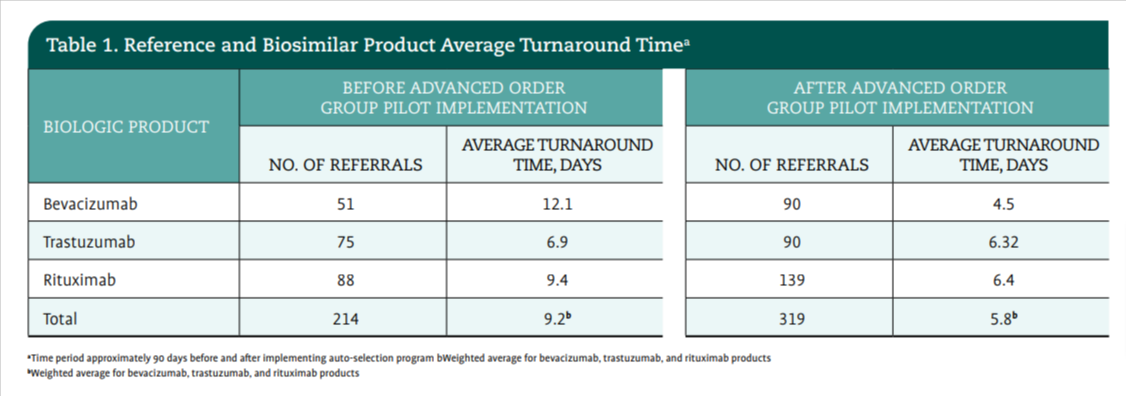
The pilot was launched with 1 reference product (bevacizumab [Avastin; Genentech]), and the 2 biosimilars that were available on the market at the time (bevacizumab-bvzr [Zirabev; Pfizer]) and bevacizumab-awwb [Mvasi; Amgen Biosimilars]). The James conducted a pre-pilot evaluation of new patient treatment plans containing the agent bevacizumab and the time between when the plan was placed in the patient’s chart and when that authorization was given. A total of 52 new plans were placed between May 2021 and July 2021. The turnaround time for authorization approval was an average of 12.1 days.
For the pilot program that ran from August 2021 through December 2021, the advanced order group rule was applied for the reference product (bevacizumab) and the 2 biosimilars. At the time of the pilot, the 2 biosimilars were preferred agents for 3 different payers. During the pilot, the advanced order group was applied to 92 new treatment plans containing bevacizumab. The turnaround time for authorization approval decreased by 36% to an average of 4.5 days to approval (Table 1). The pilot effectively provided data that the time to authorization could be reduced for payers with a preferred biosimilar.
Sustaining the Change and Expanding Solutions
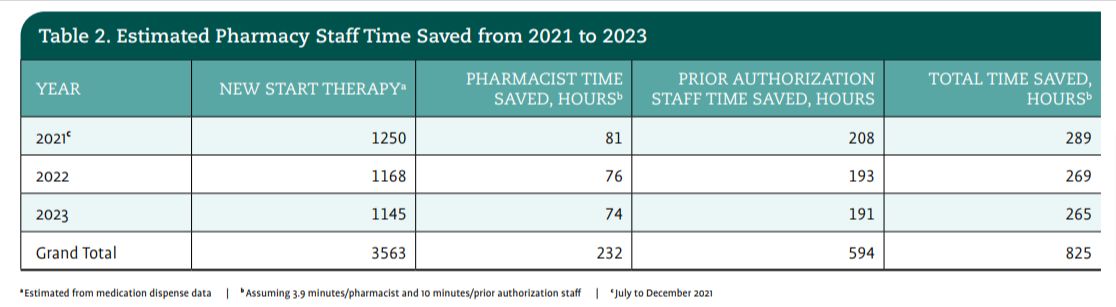
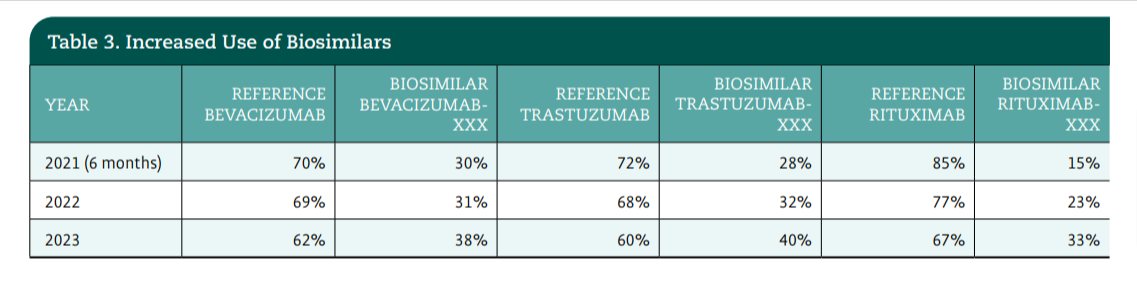
During the next phase of the pilot, the team shifted its focus to expanding the advanced order group to other biosimilars. In January 2022, The James launched 3 additional biosimilar advanced order groups for rituximab, trastuzumab, and infliximab with similar successful decreases in turnaround time and positive feedback from provider, pharmacy, and prior authorization teams (Table 2). The implementation of the biosimilar selection tool resulted in a decrease in prior authorization time and an increase in efficiencies for patient starts of therapy; this addressed the administrative burdens for providers, pharmacists, and prior authorization staff (Table 3).
Another goal of the advanced order group pilot was to increase biosimilar use at The James. From the beginning of the pilot in 2021, increased use of biosimilars provided a unique challenge, since the reference product is what is used to populate all treatment plans. When The James initiated evaluation of the selected preferred agent, a noticeable increase in use of biosimilars occurred through fiscal year 2022 and 2023 (Table 3).
The advanced order group tool for IV iron replacement provides a suggested product to providers that aligns with payer-preferred products and the most favorable margin for The James.
Sustaining the Work
The EHR-based informatics tool requires monthly and quarterly management to ensure the best results. For example, the prior authorization team manager reviews the payer preference list to monitor for any updates to preferred payer changes in biosimilars. The pharmacy business operations team gathers monthly and quarterly contract and purchasing values for the medications that are compared within the biosimilar calculator. When new information is obtained, a monthly comparison is conducted to determine the optimal medication based on payer preference and financial benefit. The list of preferred payer medications—which is stored on a shared site as a reference for clinical teams—is then updated based on this information. The final step in this ongoing process is submission of a ticket request to the pharmacy informatic teams to update and change the advanced order group for biosimilar preferences.


The process has encountered development and maintenance challenges. Timely information from payers about preferred medication choices can be delayed, and the prior authorization team has sometimes discovered changes after they have been implemented by payers. Lack of uniformity in policies to locate preferred medications on payer websites mandate a manual review that may cause user frustration and delays. Drug shortages and supply chain challenges have also impacted advanced order group preferences, resulting in the primary preference agent being removed and information on a secondary preferred agent being updated within the advanced order group. Finally, changes to the payer preference list can be delayed due to competing priorities for the pharmacy informatics team, which updates the advanced order group, or pharmacy business operations, which assesses contracting and purchasing data.
Additional Applications
With the success of its biosimilar calculator and EHR-based informatics tool, The James evaluated whether the target selection process and the use of the advanced order group would benefit the use of other pharmaceutical classes based on payer formulary preferences. For example, development of a similar advanced order group tool was applied to intravenous (IV) iron replacement. Like biosimilars, certain IV replacement iron products are given only after insurance payers apply step therapy. Policies were not uniform across payers and were causing provider and patient frustration and treatment delays. The advanced order group tool for IV iron replacement provides a suggested product to providers that aligns with payer-preferred products and the most favorable margin for The James.
Conclusion
The innovative approach displayed in the development of this technology solution highlights the opportunities for health care systems to leverage EHR solutions and for informatic pharmacists, finance, and frontline staff to collaborate. This cooperative approach serves as a model for other health care systems to successfully implement an advanced order group application. This strategy allows a preferred insurance product to be selected when treatment is planned and an order to be entered based on the patient’s insurance coverage and the health system’s payer contract arrangements.
Sarah Hudson-DiSalle, PharmD, RPh, FACCC, is assistant director of reimbursement services at The Ohio State University Comprehensive Cancer Center—Arthur G. James Cancer Hospital and Richard J. Solove Research Institute in Columbus, Ohio.
References
1. Giuliani J, Bonetti A. The economic impact of biosimilars in oncology and hematology: the case of trastuzumab and rituximab. Anticancer Res. 2019;39(7):3971-3973. doi:10.21873/anticanres.13552
2. Al-Sabbagh A, Olech E, McClellan JE, Kirchhoff CF. Development of biosimilars. Semin Arthritis Rheum. 2016;45(5; suppl):S11-S18. doi:10.1016/j.semarthrit.2016.01.002
3. Biosimilar product regulatory review and approval. FDA. Accessed October 2, 2024. https://www.fda.gov/files/drugs/published/Biosimilar-Product-Regulatory-Review-and-Approval.pdf
4. Pettit C. A level playing field for biosimilar success. Morning Consult. December 18, 2018. Accessed December 12, 2024. https://morningconsult. com/opinions/level-playing-field-biosimilar-success/
5. Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. RAND. October 23, 2017. Accessed December 12, 2024. https://www.rand.org/pubs/perspectives/ PE264.html
6. Jeremias S. FDA draft guidance removes switching study requirements for biosimilar interchangeability. AJMC | The Center for Biosimilars. June 20, 2024. Accessed December 12, 2024. https://www.centerforbiosimilars.com/ view/fda-draft-guidance-removes-switching-study-requirements-forbiosimilar-interchangeability
7. Pestaina K, Pollitz K. Examining prior authorization in health insurance. KFF. May 20, 2022. Accessed December 12, 2024. https://www.kff.org/ policy-watch/examining-prior-authorization-in-health-insurance/
8. Akosa AN. Precertification, denials and appeals: reducing the hassles. Fam Pract Manag. 2006;13(6):45-48. https://pubmed.ncbi.nlm.nih.gov/16813121/
9. 2023 AMA prior authorization (PA) physician survey. American Medical Association. Accessed August 11, 2023. https://www.ama-assn.org/system/ files/prior-authorization-survey.pdf
10. Gifford K, Winter A, Wiant L, Dolan R, Tian M, Garfield R. How state Medicaid programs are managing prescription drug costs: results from a state Medicaid pharmacy survey for state fiscal years 2019 and 2020. KFF. April 2020. Accessed December 12, 2024. https://files.kff.org/attachment/ How-State-Medicaid-Programs-are-Managing-Prescription-Drug-Costs.pdf
11. Resneck JS Jr. Refocusing medication prior authorization on its intended purpose. JAMA. 2020;323(8):703-704. doi:10.1001/jama.2019.21428
12. Chino F, Baez A, Elkins IB, Aviki EM, Ghazal LV, Thom B. The patient experience of prior authorization for cancer care. JAMA Netw Open. 2023;6(10):e2338182. doi:10.1001/jamanetworkopen.2023.38182
13. Howell S, Yin PT, Robinson JC. Quantifying the economic burden of drug utilization management on payers, manufacturers, physicians, and patients. Health Aff (Millwood). 2021;40(8):1206-1214. doi:10.1377/ hlthaff.2021.00036
14. Prior authorization and utilization management reform principles. American Medical Association. Accessed July 30, 2024. https://www. ama-assn.org/system/files/principles-with-signatory-page-for-slsc.pdf
15. Trapani D, Kraemer L, Rugo HS, Lin NU. Impact of prior authorization on patient access to cancer care. Am Soc Clin Oncol Educ Book. 2023;(43):e100036. doi:10.1200/EDBK_100036
16. Linnerooth S, Penley B, Sauvageau G, et al. Methodology for conducting a comprehensive product review in managed care. J Manag Care Spec Pharm. 2023(3):237-243. doi:10.18553/jmcp.2023.29.3.237
17. AMCP Partnership Forum: optimizing prior authorization for appropriate medication selection. J Manag Care Spec Pharm. 2020;26(1):55-62. doi:10.18553/jmcp.2020.26.1.55
18. Jensen CJ, Tichy EM, Lempke MB, et al. Implementing and optimizing biosimilar use at Mayo Clinic. Mayo Clin Proc. 2022;97(6):1086-1093. doi:10.1016/j.mayocp.2022.02.015















