A Sustainable Model for Pancreatic Cyst Surveillance and Early Pancreatic Cancer Detection
Author(s):
Mucinous cysts of the pancreas represent the most common identifiable precursor to pancreatic cancer. Evidence-based guidelines for screening and surveillance of these cysts exist; however, many patients are either not properly identified or lost to follow-up. Artificial intelligence, specifically computational linguistics models, can dramatically improve patient identification and mitigate risk through modernizing pancreatic cyst longitudinal surveillance. In this article, we discuss the risk associated with mucinous cysts of the pancreas, our modern approach to patient identification and surveillance, and outcomes from our pancreatic cyst program, as well as a pilot study of an unintended outcome, the identification of pancreatic and peri-pancreatic malignancies.
In 2023, pancreatic cancer represented the tenth and eighth most commonly diagnosed cancer in men and women, respectively.1 However, pancreatic cancer was the third to fourth leading cause of cancer-related mortality.2 More than 60000 Americans were expected to be diagnosed with pancreatic ductal adenocarcinoma (PDAC) in 2024, with more than 51000 estimated deaths.3 Moreover, the median survival for a resected and multimodal-treated PDAC remains 48 months, with a 5-year survival rate of 12% for all-comers.4 Due to these statistics, we have placed efforts on early identification and intervention.
Cooperman Barnabas Medical Center is a 697-bed hospital in Livingston, New Jersey, and a flagship for RWJ Barnabas Health and Rutgers Cancer Institute, the state’s only National Cancer Institute- designated Comprehensive Cancer Center. In partnership with RWJ Barnabas Health and Rutgers Cancer Institute, Cooperman Barnabas Medical Center focused efforts on preventive medicine, specifically improving the quality of care for patients at risk for developing pancreatic cancer. To do so, the medical center partnered with Eon and pioneered an innovative AI-based approach to automatically identify patients with pancreatic cysts, move them into evidence-based surveillance, and identify pancreatic cancer as early as possible or even intervene on a patient before pancreatic cancer develops. This initiative was inspired by the success of the Cooperman Barnabas Medical Center Incidental Pulmonary Nodule Program, which used a computational linguistics AI model to detect and manage patients at risk of developing lung cancer.
Risk Assessment
When assessing risk for the development of pancreatic cancer, non- modifiable and modifiable risk factors have been identified. Factors that cannot be mitigated include age (median age at diagnosis is 70 years), male sex, race, family history of pancreatic cancer, and inherited genetic alterations such as BRCA1, BRCA2, PALB2, FAMMM (p16/ CDKN2A), familial pancreatitis (PRSS1), HNPCC, and Peutz-Jeghers syndrome (STK11).5 However, factors that may be altered, leading to decreased risk, include tobacco use, obesity, pancreatitis, diabetes, and active management of pancreatic cysts.5 Although the propensity to develop a mucinous pancreatic cyst cannot be altered, the identification and appropriate management certainly can. Due to improvements in modern imaging quality, currently 2.3% of abdominal CT scans and 19.6% of abdominal MRI exams will incidentally identify a pancreatic cyst.6 These data are in concordance with the American Gastroenterological Association, which has stated that upward of 15% of Americans harbor a pancreatic cyst.7 Although certain pancreatic cysts can be benign, such as serous cysts or pseudocysts, intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms represent premalignant tissue and were the focus of our program.
Numerous challenges are encountered when assessing patients with mucinous pancreatic cysts. First, mucinous cysts are premalignant mucin-producing epithelial tumors that arise from the pancreatic ductal system. Second, progression from low-grade dysplasia to high-grade dysplasia to invasive cancer is the course followed by up to 30% of PDACs.8 Most experts believe that intraductal papillary mucinous neoplasms represent a field defect of the pancreas and the risk is not only in the area of radiographic IPMN. Pulling this information together, the crux for pancreatic care teams is the fact that they are faced with the dilemma between recommending complex surgery, which carries morbidity and mortality risk for low-risk IPMN, or recommending observation for those who could possibly be harboring a radiologically occult malignancy. Therefore, patients with pancreatic cysts need modern identification and surveillance strategies.
Based on the above data, mucinous cysts of the pancreas are premalignant and require appropriate identification and longitudinal surveillance. However, this disease remains one with a paucity of knowledge within the medical community. Nationally, innumerable patients each year develop PDAC in the setting of pancreatic cystic disease due to a lack of identification and evidence-based follow-up. The challenges caring for patients with pancreatic cysts start at the initial scan. Being that most pancreatic cysts are incidentally identified, most patients are never referred for further surveillance. Data suggest this number is at least 53% of patients nationally.9 Even in the select patients who are referred, most programs use manual patient entry into Excel spreadsheets, which lack patient demographics and clinical correlates; patient compliance for the return for screening can be low; clinical data are fragmented; and an individual program lacks the ability to submit to a national registry. In summary, a significant number of patients with incidental pancreatic cysts are not identified; identified patients are not referred; referred patients are manually tracked; and patient data are not entered into a database repository to ask quality and research questions on a population level. In addition, existing health care disparities may limit access to surveillance programs for lower socioeconomic populations.
Modernizing Pancreatic Cyst Identification and Surveillance
Cooperman Barnabas Medical Center partnered with Eon (https:// eonhealth.com, Denver, Colorado) to design a highly flexible software system utilizing computational linguistics models and a codebook specific to pancreatic imaging. Eon Patient Management (EPM) software integrates with the electronic health record (EHR) and facilitates patient identification, risk assessment, care plan setting, care plan tracking, patient and provider communication, outcome recording, and registry functionality. EPM software covers incidentally and screening-detected findings across a broad array of organ systems. Using computational linguistics data models, EPM software can accurately identify and capture pancreatic abnormalities from radiology reports with 97.5% accuracy on multiple radiology modalities, including CT, MRI, and ultrasound. Also, EPM software automates mundane and repetitive tasks such as sending letters and tracking if appointments have been made. The advantages to using this AI software will be discussed in 2 categories: identification and surveillance.
Regarding identification, patients with pancreatic cysts and their respective demographics are electronically populated from the EHR into the EPM dashboard. To accomplish this task, radiology reports are analyzed through the computational learning model, patients identified with pancreatic cysts are added to the EPM worklist so they can be tracked, and management decisions are captured. Following identification at our institution, patients are contacted by letter and phone call from a nurse navigator and offered consultation with one of our pancreatic surgeons. The ordering provider is also sent a letter explaining the identification and the pancreatic cyst surveillance identification program. If the patient elects to be seen, consultation is scheduled at that time. The use of the AI computational linguistics model, therefore, mitigates patient loss and prevents a lack of follow-up options for a possible premalignant lesion of the pancreas.

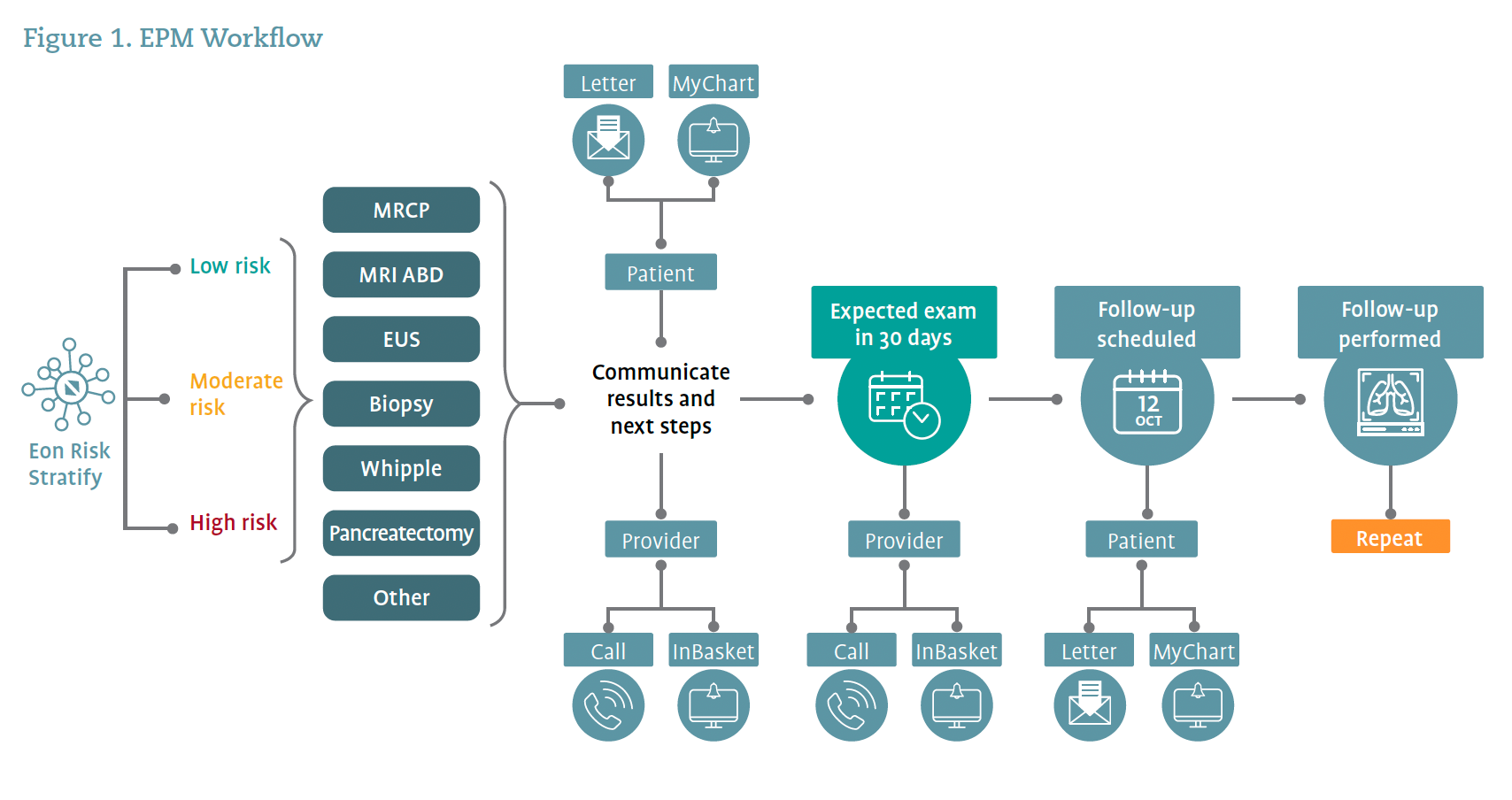
For longitudinal surveillance, a cloud-based EPM dashboard replaces the traditional and antiquated Excel spreadsheet. The dashboard electronically monitors orders and appointments for the patients on the worklist. Thus, the dashboard not only shows patients identified and patients seen, but also highlights upcoming events, patients who are at risk for missing follow-up, and documents the follow-up advised by the pancreatic surgeon. Phone call reminders or reminder letters to patients can be programmed, and reminders for surveillance imaging and time points can be set to ensure that exams and procedures are scheduled and performed. The automation rules and electronic monitoring make it easy for the care team to identify patients who have missed their interval imaging, endoscopy, or surveillance appointment because the EPM software is organized into work lists to ensure patients are not lost to follow-up. On a patient level, the dashboard allows the care team to see the cyst details longitudinally to assess for concerning change and can therefore perform real-time risk stratification. Overall, the EPM software automates repetitive tasks, allowing patient coordinators to spend more time on patient care and less time on administrative duties. Use of the software ensures that patients are tracked and followed according to published evidence-based guidelines. Figure 1 illustrates the EPM workflow.
This AI platform allows Cooperman Barnabas Medical Center to adopt workflows to ensure seamless management and surveillance of patients with pancreatic cysts. As many as 53% of patients with incidental pancreatic cysts are lost to follow-up because their cysts are not identified, identified patients are not referred, or referred patients are manually tracked and lost to the system.9 Besides the negative impact on patient outcomes, this process also means patient data are not entered into a database repository to ask quality and research questions on a population level to advance our knowledge of this cancer.
Key to the success of the EPM workflow is the platform’s ability to automate steps along the patient journey based on the risk profile of the pancreatic finding and the patient’s anticipated adherence to the care plan to avoid unnecessary notifications. The software is managed by exception, meaning that both communication cascades and the nurse navigator’s dashboard focus attention on patients at risk for non-adherence. Patients who are on track for expected care, as well as their physicians, receive a baseline level of communication, and those patients are suppressed from the nurse navigator’s view because they do not require action. Multimodal outreach is used for those patients not meeting key milestones and that information is stored in the EHR. The AI platform dynamically assesses the patient and their medical record until they complete the next expected step in their care plan and updates communication and the care plan as new information is received.
In summary, patients with mucinous pancreatic cysts require lifelong surveillance. AI software has modernized the identification, capture, and longitudinal management of these patients, allowing more patients to receive evidence-based, high-quality management and not be lost to follow-up.


Clinical Outcomes of the Pancreatic Cyst Surveillance Program
Cooperman Barnabas Medical Center’s pancreatic cyst surveillance program achieved its 2 primary goals: 1) the identification of incidental pancreatic cysts/lesions and 2) the longitudinal surveillance of at-risk patients (Figure 2). The program currently manages more than 1000 patients, with a greater than 375% increase in new patients requiring follow-up since the adoption of the AI platform. To date, 17% of identified pancreatic cysts are classified as high risk using current consensus guidelines and sometimes require more significant intervention. The rest of these patients will be monitored to track the progression, or lack thereof, of their pancreatic cysts, as data show that pancreatic cysts growing more than 5 mm in a year have a 24% higher risk of malignant progression. The inherently longitudinal nature of this disease means that the 1420 unique patients identified in the pilot study period generated 1300 downstream procedures, such as MRIs and endoscopic ultrasounds (Figure 2).

An Unintended Secondary Outcome of the AI Solution
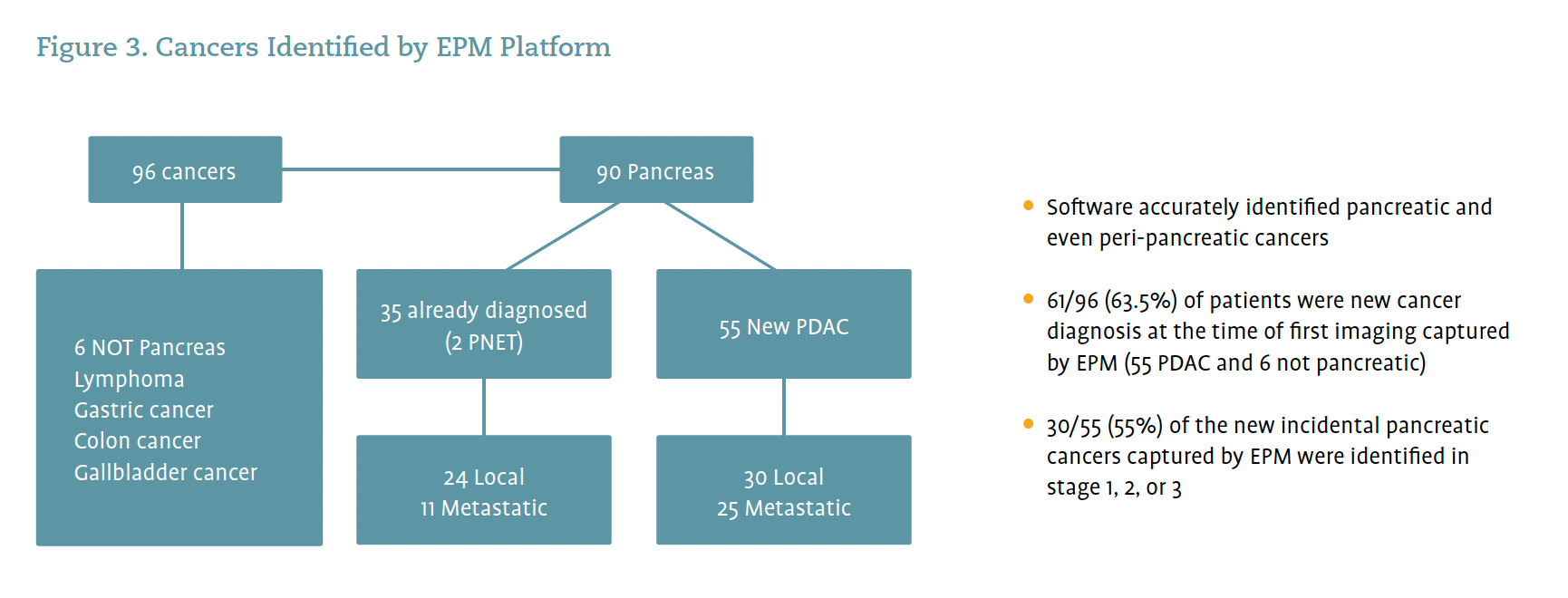
There are promising data to indicate that the platform’s ability to identify pancreatic risk extends beyond its initial goal of identifying pancreatic cysts to enable the identification of cancers. The result is that the AI software is now pulling patients with cancer, not just patients with pancreatic cysts, into the program (Figure 3). The EPM software identified 96 cancers in a retrospective review, of which 6 were not pancreatic. The 90 pancreatic cancers consisted of 35 already diagnosed patients and 55 patients whose pancreatic cancer was incidentally discovered at the time of first imaging captured by the EPM software (Figure 3). The EPM software also identified ampullary adenoma in a patient with high-risk features; the final pathology after surgery found the ampullary adenoma to contain high-grade dysplasia.
While further research is required, it is noteworthy that most of the newly diagnosed pancreatic cancers were identified before stage 4. These data stand in contrast to the national averages discussed above. It is possible that this stage distribution is owed to socioeconomic factors that could not be understood given the limited scope of the research, and ongoing analysis is planned.
Concluding Thoughts
The pancreatic cyst surveillance program can serve as a model for other organizations looking to change the paradigm for patients at risk of developing pancreatic cancers by monitoring pancreatic cysts to enable early intervention. The AI-driven approach described in this article has enabled Cooperman Barnabas Medical Center to increase the number of patients under surveillance significantly and sustainably while delivering the key benefits of:
- Mitigating health disparities because the software works consistently for all patients and captures at-risk patients who may be using the emergency department as a stand-in for primary care
- Improving the quality of care of patients living with malignancy risk by enabling longitudinal monitoring and proactive patient management through intelligent automation
- Improving medical and legal risk for physicians and care teams by ensuring that patients do not fall through the cracks.
Lastly, our initial analysis indicates that the AI software accurately identifies pancreatic and possibly peri-pancreatic cancers, which may allow for expeditious cancer care pathways.
Russell C. Langan, MD, FACS, FSSO, is associate chief surgical officer, System Integration and Quality & director of Surgical Oncology, RWJBarnabas Health, Rutgers Cancer Institute of New Jersey, Cooperman Barnabas Medical Center in Livingston, New Jersey.
References
- American Cancer Society. Key statistics for pancreatic cancer. Updated January 16, 2025. Accessed February 7, 2025.https://www.cancer.org/cancer/types/pancreatic-cancer/about/key-statistics.html
- National Cancer Institute. Cancer stat facts: pancreatic cancer. Accessed February 7, 2025. https://seer.cancer.gov/statfacts/html/pancreas.html
- American Cancer Society. Cancer facts & figures 2024. Accessed February 11, 2025. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2024-cancer-facts-figures.html
- Fan M, Deng G, Ma Y, Si H, Wang Z, Dai G. Survival outcome of different treatment sequences in patients with locally advanced and metastatic pancreatic cancer. BMC Cancer. 2024;24:67. doi:10.1186/s12885-024-11823-8
- American Cancer Society. Pancreatic cancer risk factors. Updated February 5, 2025. Accessed February 7, 2025. https://www.cancer.org/cancer/types/pancreatic-cancer/causes-risks-prevention/risk-factors.html
- Zhu S, Wang WT, Shang XS, et al. Difference analysis in prevalence of incidental pancreatic cystic lesions between computed tomography and magnetic resonance imaging. BMC Med Imaging. 2019;19:43. doi:10.1186/s12880-019-0341-5
- American Gastroenterological Association. Diagnosis and management of asymptomatic neoplastic pancreatic cysts. Published February 25, 2015. Accessed February 7, 2025. https://gastro.org/clinical-guidance/diagnosis-and-management-of-asymptomatic-neoplastic-pancreatic-cysts
- Del Chiaro M, Segersvärd R, Lohr M, Verbeke C. Early detection and prevention of pancreatic cancer: Is it really possible today? World J Gastroenterol. 2014;20(34):12118-12131.doi:10.3748/wjg.v20.i34.12118
- Megibow AJ, Baker ME, Morgan DE, et al. . Management of incidental pancreatic cysts: a white paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2017;14(7):911-923. doi:10.1016/j.jacr.2017.03.010















